
The new standard in
manual reprocessing
using wipes
The new standard in
manual reprocessing
using wipes
Professional reprocessing of medical devices is a challenge in the everyday routine of a hospital or practice. To provide the best possible support, we have developed the gigasept® powerSET3: easy-to-use, safe, well thought-out and comprehensively effective.
You're on the safe side
To exercise due care even when time is pressing, we offer the gigasept® powerSET3 for wipe-based reprocessing of probes and endoscopes without lumen. The gigasept® powerSET3 is a solution you can rely on.
Benefits for ...
… patients: a clean and disinfected medical device
… users: easy handling, seamless documentation, maximum process reliability, space-saving design
The powerful trio of cleaning, disinfection and rinsing:


gigasept® powerSET3 – for reprocessing of the following non-lumened medical devices*:

Transvaginal
ultrasound probes
(TVUS)

Transoesophageal
echocardiography probes
(TEE/TOE)

Transrectal
ultrasound probes
(TRUS)

Non-invasive
ultrasound
probes

Flexible endoscopes
without channels (e.g.
nasopharyngoscopes)
* Please follow the reprocessing recommendations provided by the medical device manufacturers. Not suitable for reprocessing of critical medical devices.
More power, less effort

Convince yourself of the efficiency and easy handling.
This video shows the correct application of gigasept® powerSET3.
Reprocessing is a matter of trust
The optimum formula is used for cleaning, disinfection and rinsing in gigasept® powerSET3. Furthermore, we use high-quality non-woven fabric (lint-free) for reliable cleaning and even wetting.
- Cleaning wipe: special surfactant formula for reliable cleaning of residues after removal of any protective covers
- Disinfection wipe: antimicrobial solution based on peracetic acid for high-level disinfection
- Rinsing wipe: deionised water for safe removal of possible disinfectant residues
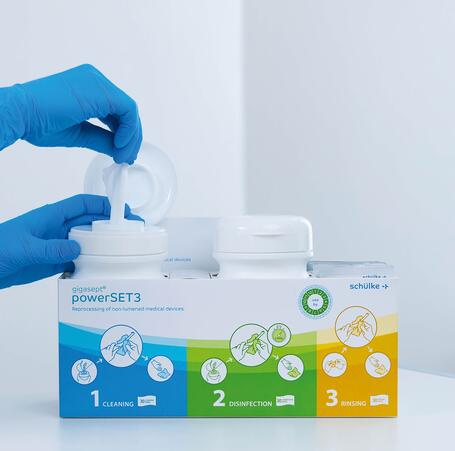
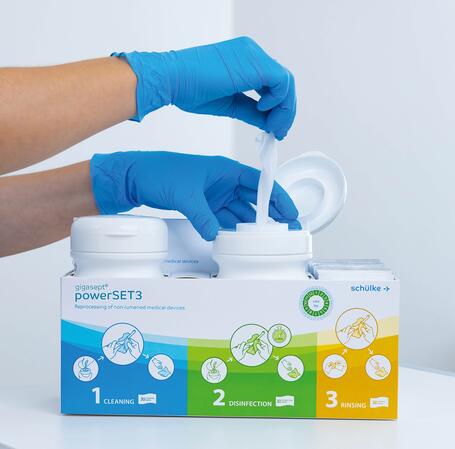
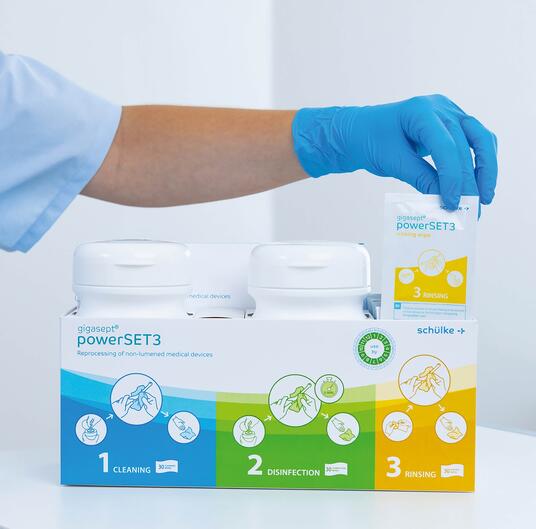
Optimal support, maximum safety
Safety is paramount with gigasept® powerSET3. Therefore, we designed the gigasept® powerSET3 to provide users with the best possible support. With our solution, only the absolutely necessary steps need to be carried out. This saves time and prevents errors.
The wipes are fully prepared, ready-to-hand and arranged logically from left to right in the box. The two tubs are firmly attached to the box to prevent mix-ups. In addition, the surface of the box has an antimicrobial coating (Lock 3) to protect against cross-contamination.

Support documentation, reduce workload
Documentation is also simplified using gigasept® powerSET3. A documentation book is enclosed in each box of gigasept® powerSET3.
- Documentation book clearly assigned to the box via a common batch number
- Checkboxes support the documentation process
- Stickers replace handwritten notes

Validation tested under conditions close to actual practice
In collaboration with an accredited testing laboratory, we successfully conducted hygienic-microbiological assessments on different medical devices (transvaginal, transrectal and TEE probes). We have thus demonstrated that the entire process can be reliably reproduced and validated using the gigasept® powerSET3.*
- Use of different probes with hard-to-reach areas and large surfaces
- Practical conditions for reproducible results
- Validation of cleaning and disinfection
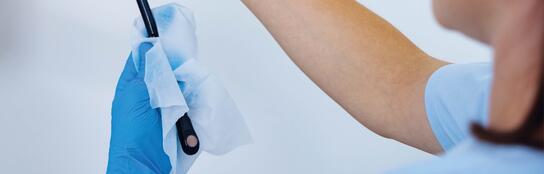
* Reprocessing must be validated according to the individual conditions on site.
Because every step counts
The gigasept® powerSET3 also helps to reduce waste. Compared to three-component systems with individually packed wipes, it generates considerably less plastic waste.


Comprehensive efficacy within short period
The antimicrobial efficacy of the gigasept® powerSET3 disinfection wipe has been proven by all required testing according to the current version of EN 14885 and thus corresponds to the state of the art.
| Efficacy | Test method | Contact time |
| Bactericidal | EN 13727 / EN 14561 / EN 16615 / VAH | 1 Min. |
| Yeasticidal | EN 13624 / EN 14562 / EN 16615 / VAH | 1 Min. |
| Mycobactericidal | EN 14348 / EN 14563 / EN 16615 | 5 Min. |
| Fungicidal | EN 13624 / EN 14562 / EN 16615 | 5 Min. |
| Virucidal | EN 14476 / EN 16777 / EN 17111 / EN 16615 (mod.) / DVV/RKI | 5 Min. |
| Polyoma SV40 | EN 16615 (mod.) / DVV/RKI | 1 Min. |
| Sporicidal (C. difficile) | EN 17126 / EN 17846 / VAH | 5 Min. |
Award-winning design
The gigasept® powerSET3 has already been recognised: winner of the German Packaging Award 2022 in the category "Functionality & Convenience" as well as winner of the WorldStar Global Packaging Award 2023 in the category “Medical and Pharmaceutical”.


Contact
Schülke & Mayr GmbH
Robert-Koch-Str. 2
22851 Norderstedt I Deutschland
Telefon: +49 40 52100-0
Telefax: +49 40 52100-660
E-Mail: nfschlkcm
Contact form - your direct line to schülke
What can we do for you? Simply fill out the following form and we will get back to you.
Data protection notice
We use analysis methods (e.g. cookies) to measure how often our website is visited and how it is used. We also use cookies to link your page visits and website usage with your customer data stored in our CRM system in order to be able to address you individually, i.e. based on your interests and usage. We alsowe embed third-party content from other providers (e.g. videos). We have no influence on further data processing and any tracking by the third-party provider.In this context, we also use service providers in third countries outside the EU that may not guarantee an adequate level of data protection, which entails the following risks Access by authorities without informing the data subject, no data subject rights, no legal remedies, loss of control.By hiring us, you consent to the processes described above. You can revoke your consent with effect for the future. You can find more information in our privacy policy


















